Why are the electron affinities of beryllium and magnesium is almost zero ?? Give five reason? ?
1 Answer
Here's my explanation.
Explanation:
Electron affinity
Electron affinity
#"A(g) + e"^"-" →"A"^"-""(g)"#
The higher an atom's tendency to accept an electron, the more positive the electron affinity value will be.
Beryllium and magnesium
The general trend is that electron affinity increases from left to right in the Periodic Table.
Unlike most elements, they have
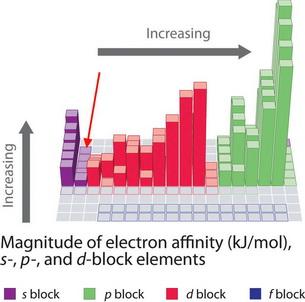
(Adapted from FlatWorld)
Why are beryllium and magnesium so different?
-
#"Be"# and#"Mg"# have filled#"s"# ubshells. They are much more stable than if they had an extra electron. -
An extra electron would have to go into a
#"p"# orbital, which is at a much higher energy than an#"s"# orbital. We must add energy to put an electron into a higher energy subshell, so#E_text(ea) ≤ 0# .
I could probably construct three more reasons for the difference, but they would be stating the above comments in different words.

