Why BeF2 molecule has zero dipole moment although the Be-F bonds are polar.....?
1 Answer
Mar 12, 2018
Dipole moments cancel out.
Explanation:
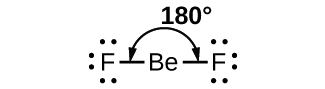
In this case, fluorine is much more polar than beryllium, with an electronegativity difference of
However, there is an opposite fluorine atom on the other side, pulling at the same strength, and as we know in physics, if two balanced forces are pulling in different directions, then no movement happens.
So here, beryllium atoms are pulled at same strengths in different directions, and so they do not move at all, thus canceling out the dipole moments.
I hope this helps!

