Why do alkyl groups have lower electronegativity versus hydrogen? How does this affect the acidity or alkalinity of alcohols?
1 Answer
At first glance, I think you were referring to hyperconjugation, and wondering about how
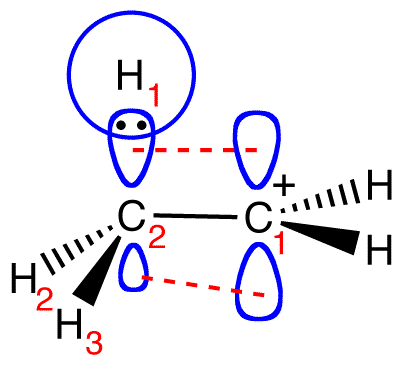
HYPERCONJUGATION OF CARBOCATIONS
In general, delocalized negative charge stabilizes a molecule.
Since the central
The alkyl
 http://www.ochempal.org/
http://www.ochempal.org/
Having more alkyl groups surrounding the central carbon thus stabilizes it more than having fewer.
(This is not, however, related to the electron-donating capability of alkyl groups. It is merely a result of removing a substituent and generating a positive charge, which generally makes the compound less stable anyways.)
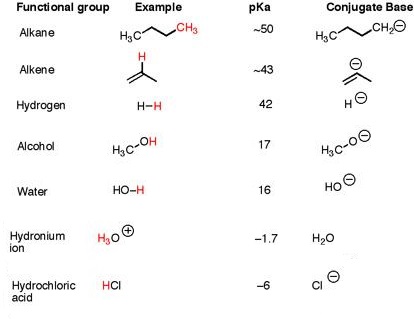
ACIDITY OF ALKANES
Alkanes have a high
"RCH"_3 larr "RCH"_2^(-) + "H"^(+)
If one manages to deprotonate an alkane, then what would result is an exceptionally great (Lewis) base, whose electron density also happens to be extremely localized onto the frontal carbon atom, allowing for easy electron donation.
(Great nucleophilicity is the ability to gather electron density easily and donate it quickly, but great basicity is the thermodynamic instability of the compound in question.)
ACIDITY OF ALCOHOLS
Analogizing with alkanes, alcohols have hydroxyl groups (

(The structural difference is that an oxygen atom is inserted between the terminal carbon's
That means the electron density is localized more in the
 https://s-media-cache-ak0.pinimg.com/
https://s-media-cache-ak0.pinimg.com/
Therefore, alcohols are (much) more acidic than alkanes.
This is reflected in the

