Why do different elements make different color flames when you burn them?
1 Answer
Different elements have different flame colours because their electrons have different allowed energy levels.
Explanation:
The Bohr model says that electrons exist only at certain allowed energy levels.
When you heat an atom, some of its electrons are "excited* to higher energy levels.
When an electron drops from one level to a lower energy level, it emits a quantum of energy.
The wavelength (colour) of the light depends on the difference in the two energy levels.
We can see only those transitions that correspond to a visible wavelength.
In a hydrogen atom, for example, we can see only the transitions from higher levels to n = 2 (the Balmer series).

Every element has its own characteristic set of energy levels.
Thus, an atom of Na has different energy levels and transitions than an atom of Li.
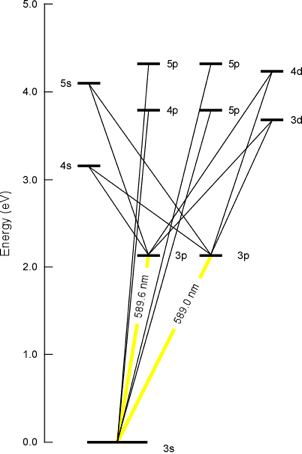
The different mix of energy differences for each atom produces different colours.
Each metal gives a characteristic flame emission spectrum.

Check out these videos of flame tests...
videos from: Noel Pauller


