Why do ionization energies increase from left to right across any period?
1 Answer
Dec 5, 2015
Because of increasing nuclear charge,
Explanation:
While going from left to right, we are adding electrons, as well as protons, electrons are entering the same shell, and the electrons do not shield each other effectively. The valence electrons experience an effectively larger nuclear charge going to the right of the Period. Ionization energies should progressively increase. Do they?
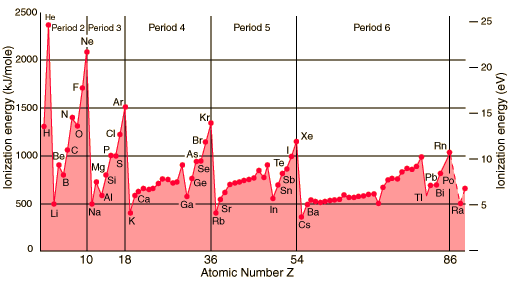
Ionization Energy
hyperphysics.phy-astr.gsu.edu512 × 285Search by image
What do you think the above effect will have on ATOMIC size?

