Why do molecules have the names like (2E)-3-Phenylprop-2-enal?
1 Answer
Jan 17, 2018
There are so many organic molecules that we need a systematic way to give each one a unique name.
Explanation:
The International Union of Pure and Applied Chemists (IUPAC) has developed a system of rules that give each molecule a unique name.
Let's consider (E)-3-phenylprop-2-enal.
The parts of the name tell you:
- prop = the main chain of three carbons
- al = an aldehyde group (automatically at
#"C1")# - 2-en = a
#"C=C"# double bond between#"C2"# and#"C3"# - 3-phenyl = a phenyl group on
#"C3"# - E = the two alkene
#"H"# atoms are opposite to each other
There is only one arrangement of atoms that fits this name:
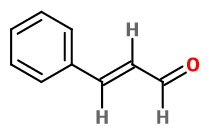
The trivial name is cinnamaldehyde, because it gives cinnamon its characteristic flavour and odour.
Think of all the problems we would have if we had to memorize the trivial names of thousands of compounds!
The IUPAC name of a molecule gives us a way to deduce its structure.

