Why do nitrogen and phosphorus have high ionisation energies?
1 Answer
Well, because they are non-metal atoms, oxidizing agents, with relatively high, unshielded nuclear charge.....
Explanation:
So let us find some data....
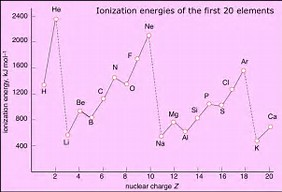
AS nuclear charge increases, the ionization energy should also increase across the Period. However, there is a DECREASE in ionization energy observed for nitrogen, and oxygen, and for phosphorus and sulfur.
And note that we interrogate the ionization reactions....
The apparent anomalous ionization energy of nitrogen versus oxygen, is probably a consequence of Hund's rule of maximum multiplicity....the spin quantum number of the nitrogen atom is maximized here, with three unpaired electrons...and the

