Why do the members of Group 1 react by losing an electron, but the members of Group 17 react by gaining an electron?
1 Answer
Because metals are reducing....
Explanation:
And non-metals are oxidizing...
Consider the electronic structure of the alkali metals. These have a SINGLE valence electron...and are readily oxidized..
On the other hand, non metals, i.e. dioxygen, difluorine, and dichlorine, have high unshielded nuclear charge, and certainly this is reflected by the atomic radii...WHICH DECREASE ACROSS a Period, from left to right as we face the Table.....
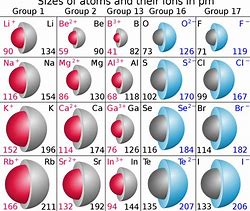
And thus the halogens have high UNSHIELDED nuclear charge, and are very readily reduced .... to give FORMALLY LARGER ANIONS, i.e.
And so the relative reactivities owe to atomic structure, which is certainly reflected by the Periodic Table....

