Why does CS2 reacts with water , but CCl4 doesn't?
1 Answer
Both react, but they need a bit of encouragement.
Explanation:
They react exceptionally slowly at room temperature.
The addition of heat and a catalyst makes the reactions much faster.
However, carbon disulfide is more reactive than carbon tetrachloride.
Carbon disulfide
Carbon disulfide reacts with water at 150 °C:
Carbon tetrachloride
The reaction with water is slow because of steric crowding by the
The chlorine atoms are so bulky and the carbon atom so small, that the oxygen has difficulty attacking the carbon atom.
Here is what a water molecule "sees" as it comes in for an
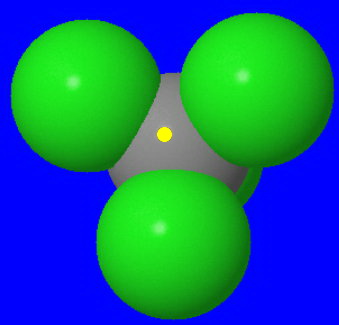
The yellow dot marks the back side of the carbon.
Even when the water molecule does get in, the trigonal bipyramidal transition state is even more crowded.
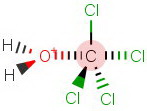
An unstable transition state means a high activation energy and a low reaction rate.
Carbon tetrachloride reacts with water in the presence of metals such as aluminum and iron.
The reaction with superheated steam does not require a catalyst.

