Why does #"NaOH"# only react with one acidic proton in salicylic acid even though both the carboxyl and the hydroxyl groups could potentially react with it?
1 Answer
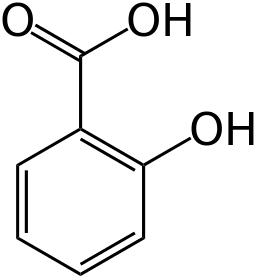
Yes, technically, salicylic acid has two acidic protons. But there is a significant difference in their acidities. Each proton has its own
This is a thermodynamic quality, and is therefore also a measure of the energetic favorability of one proton's dissociation over another for the first deprotonation.
- Carboxylic acid protons in general usually have a
#"pKa"# around#5# . - Alcoholic protons in general usually have a
#"pKa"# around#15 - 17# .
Remember that a difference of
So, it is over

