Why does nitrite have a negative charge instead of a positive charge?
So I was working on some polyatomic ions concept things and I found some confusing parts. NO2 has 17 outermost shell electrons and it has to lose 1 electron to become stable (16). I know this concept is wrong but I dont know what is the correct one. So as what I considered, NO2 should be positive 1 instead of negative 1. Please help me out with this. It will help a lot. Thank you very much and sorry for my bad English.
So I was working on some polyatomic ions concept things and I found some confusing parts. NO2 has 17 outermost shell electrons and it has to lose 1 electron to become stable (16). I know this concept is wrong but I dont know what is the correct one. So as what I considered, NO2 should be positive 1 instead of negative 1. Please help me out with this. It will help a lot. Thank you very much and sorry for my bad English.
1 Answer
Because it is the conjugate base of the weak acid
There is no specific number of electrons or any sort of electron "rule" that nitrite "follows" to become stable...
#:ddot"O"=stackrel((+))"N"=ddot"O":# ,a linear polyatomic ion instead of a bent one.
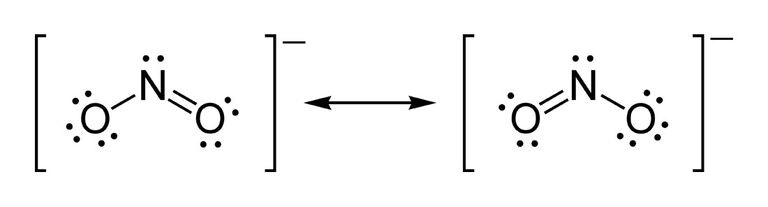 http://dziuk.info/wp-content/
http://dziuk.info/wp-content/

