Why does SiF4 act as a lewis acid?
1 Answer
Jan 4, 2016
Explanation:
A Lewis acid is an electron-pair acceptor.
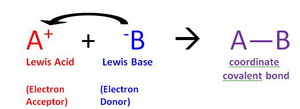
(from chemwiki.ucdavis.edu)
For example, it reacts with

A Lewis acid is an electron-pair acceptor.

(from chemwiki.ucdavis.edu)
For example, it reacts with


