Because if it wasn't, the phase change wouldn't happen.
At a specific temperature, there exist phase changes, like vaporization and melting, to transition from one phase to another (like liquid->gas or solid->liquid).
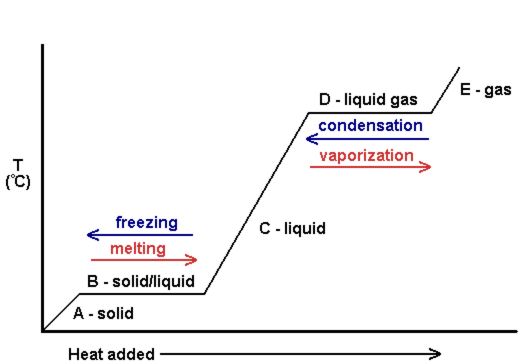
Let's consider a vaporization at #T = T_"vap"#, i.e. a vaporization at the boiling point.
A phase change is really a pressure equilibrium with the surroundings.
At #T = T_"vap"#, the vapor pressure of the liquid (directly proportional to the amount of gas currently in equilibrium with the liquid) equals the atmospheric pressure (of the surroundings), so the molecules at the surface of the liquid vaporize and float off as gases (boiling, not evaporation).
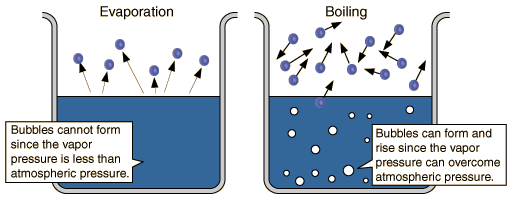
If all of a sudden, #T_"vap"# increased (or #T# decreased), then #T < T_"vap"# and we are no longer at the temperature condition required to vaporize the liquid. The vapor pressure is now not equal to the atmospheric pressure. Therefore, the liquid stops boiling and thus the phase change has stopped occurring.
This is why adding salt to boiling #"H"_2"O"#, which raises the boiling point, allows the water to go above #100^@ "C"# before boiling. Similarly, if you add salt to ice #"H"_2"O"#, it will lower the freezing point #T_"frz"# and thus melt the ice, because now #T_"frz" < T# and you've exceeded the required melting point.



