Why does the atomic radius of an element generally decrease across a period and why does it increase down a group?
1 Answer
This is a function of nuclear charge, of
Explanation:
Incomplete electronic shells shield the nuclear charge VERY INEFFECTIVELY. And this is manifested by the well known DECREASE in atomic radii from left to right as we face the Table....
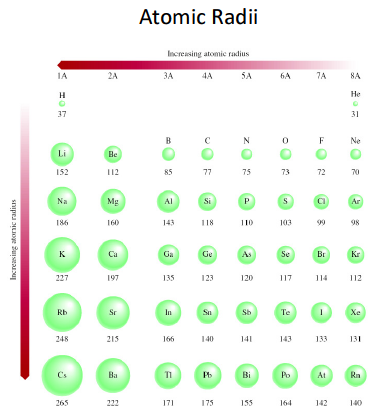
The units are in
Once a valence shell is filled, at the Noble Gases, the next electron joins the atom in A NEW VALENCE shell, that is farther removed from the nuclear core.
Of course down a
The competition between shielding and nuclear charge in large part explains the underlying structure of the modern Periodic Table.....

