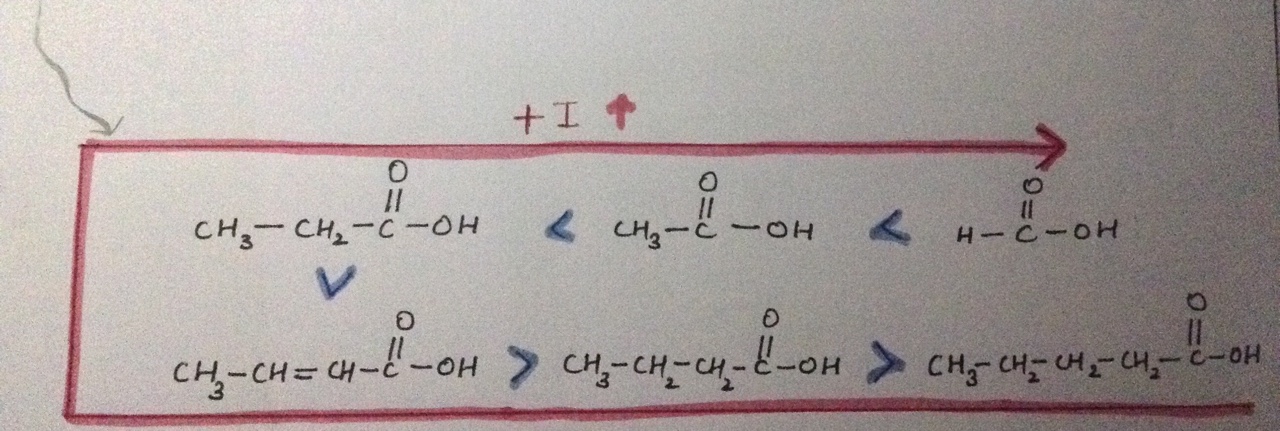
Why does the +I effect increases here?
1 Answer
The length of the carbon chain affects the acid strength of a carboxylic acid.
Explanation:
In acids, the electron-releasing
Furthermore, the strength of an acid depends on the tendency to release proton when the acid is dissolved in water.
More negative is the charge on carboxylate ion, the more is its reactivity and more is its basic nature i.e. lesser is acidic nature.
Alkyl groups are electron donating species. Thus, they increase the negative charge on the carboxylate ion (

