Why hydrogen is easily displaced by metal from acid?
2 Answers
See the explanation
Explanation:
Hydrogen is displaced by the metals from acids that are placed above hydrogen in the reactivity series of the metals.
This is because these metals are more reactive than hydrogen.
Reaction of calcium with hydrochloric acid.
Here ,
This is due to the fact that "metal acts as a reducing agent while acid acts as an oxidizing agent upon reaction".
Explanation:
Metals usually act as reducing agents in most reactions. So in case of their reactions with acids, they undergo a single replacement reaction results in the evolution of hydrogen gas and the solution of the metal which is dissolved in the acid.
For example, Magnesium metal reacts with hydrochloric acid to form a solution of magnesium chloride and hydrogen gas is evolved according to this equation:
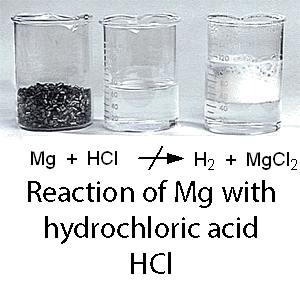
But, unfortunately not all metals can be dissolved in acids with respect to the aforementioned general idea. This is because this reaction is controlled by the "Activity Series Of Metals" in which metals are arranged vertically with respect to hydrogen element.
The essence of this order illustrates that metals that are above H in the series are more reactive that it and can expel it from acids, while the metals that are below H can't react with acids and replace hydrogen.
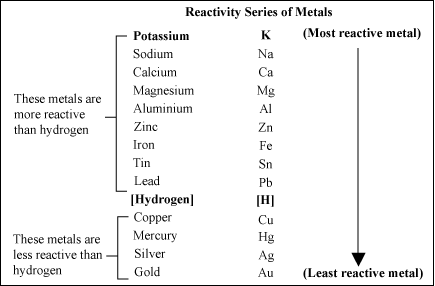
Also, you can exploit this trend of metals to expect the result of a single replacement between a metal and a solution of another metal; this is based on the fact that: the higher the reactivity of the metal is, the higher tendency it has to repel the lower reactive metal from its solution.
For example, if you put a plate or granules of Zinc into a Copper sulphate solution (blue colour), you will gain a solution of Zinc Sulphate (colourless) and an elemental Copper (red) which is precipitated.

On the other hand, if you insert a silver plate into a Zinc Sulphate solution, there is no observed reaction.


