Why is a molecule of #CH_4# nonpolar?
1 Answer
The dipoles cancel out each other, depicted in a VSEPR diagram.
Explanation:
When determining the polarity of a molecule, we use 2 methods:
#1: Electrongativity,
#2: Symmetry of molecule.
Electronegativity of a compound can be calculated by subtracting the element with the highest electronegativity with the lowest. Electronegativity can be found on the periodic table:
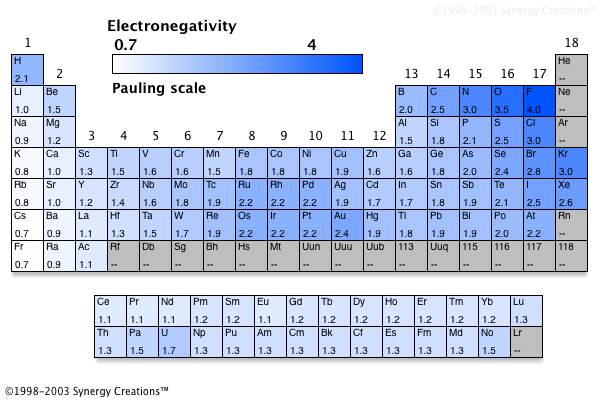
Compounds with an electronegativity of
In methane's case,
#2.5-2.1=0.4#
Disclaimer: electronegativity only determines the intramolecular bond within a compound. Not the overall compound's polarity. To do this, we use symmetry.
- Molecular Symmetry:
Molecular symmetry is the use of geometrical arrangement that can help predict the shape of molecules. These arrangements are called VSEPR: Valence Shell Electron Pair Repulsion. Lewis diagrams work too, but lacks additional/specific information that is necessary for a more "academic" standard, that a VSEPR diagram provides.
When a molecule is "symmetrical", it means the dipoles cancel. This only occurs when the outside atoms are arranged in such a way that surrounds the central atom, where the dipoles cancel - no side of the molecule has more charge (positive or negative} than the other(s).
In methane's case,
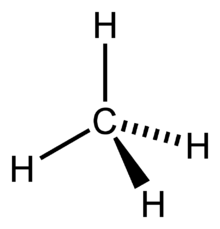
All the outer atoms are the same - the same dipoles, and that the dipole moments are in the same direction - towards the carbon atom, the overall molecule becomes non-polar.
Therefore, methane has non-polar bonds, and is non-polar overall.
Keep in mind that molecules can have polar bonds, but be non-polar overall.
Hope this helps :)

