Why is a reaction spontaneous when Delta G < 0?
1 Answer
Because that's how nature works... it wants to minimize its energy.
You should recall that achieving a minimum potential energy allows a chemical bond to be made:
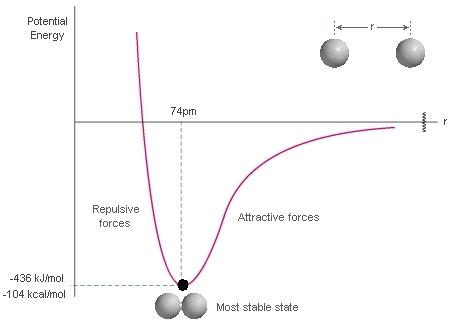 https://www.thestudentroom.co.uk/
https://www.thestudentroom.co.uk/
Just as molecules and atoms want to be at a minimum potential energy, they also want to be at a minimum chemical potential. The chemical potential is given as:
mu = G/n ,where
G is the Gibbs' free energy in"J" , andn is in"mols" .
As such, the Gibbs' free energy also wants to minimize itself, with respect to the extent of reaction,
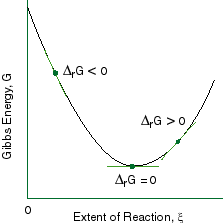 http://www.public.asu.edu/
http://www.public.asu.edu/
That is, the slope of the graph is:
barul|stackrel(" ")(" "(dG)/(d xi) harr Delta_rG" ")|
You would better know
"N"_2(g) " "+" " 3"H"_2(g) -> 2"NH"_3(g)
"I"" "n_(N_2)" "" "" "" "n_(H_2)" "" "" "n_(NH_3)
"C"" "-x" "" "" "-3x" "" "" "+2x
"E"" "n_(N_2)-x" "n_(H_2) - 3x" "n_(NH_3)+2x where
xi = x .
- If the initial state of a reaction proceeds to give a decrease in
G , then the reaction is spontaneous. But that means
(dG)/(d xi) < 0 and corresponds to
Delta_rG < 0 .That is, the reactants are still too energetic, and want to use that energy to make products.
- When
(dG)/(d xi) = 0 , it means the chemical potentials of all the reactants and products have settled, and the rate of the forward and reverse reactions are equal (though not necessarily zero).
This is dynamic chemical equilibrium.
- When
(dG)/(d xi) > 0 , it just means the process is nonspontaneous for the reaction direction we care about.
In other words, the reaction is spontaneous in the reverse direction... the products are still too energetic, and want to use that energy to make reactants.

