Why is a single pure product obtained from the hydroboration–oxidation of 2-butyne, whereas two products are obtained from hydroboration–oxidation of 2-pentyne?
1 Answer
Feb 2, 2015
The hydroboration–oxidation of but-2-yne forms a single pure product because but-2-yne is a symmetrical alkyne. Pent-2-yne forms two products because it is an unsymmetrical alkyne.
The hydroboration-oxidation of an internal alkyne forms a ketone:
R-C≡C-R' → RCH₂C(=O)R'
But-2-yne is a symmetrical alkyne. It has the same R groups (CH₃) on each end.
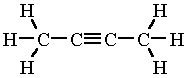
The ketone group could form either on C-2
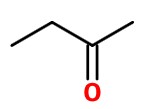
or on C-3.
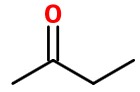
But these are these are the same compound — butan-2-one.
Pent-2-yne is an unsymmetrical alkyne. It has different R groups on each end. One end has a CH₃ group; the other end has a CH₂CH₃ group.

The ketone group could form either on C-2
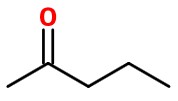
or on C-3.
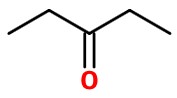
These are two different compounds. The first is pentan-2-one.The second is pentan-3-one.

