Why is cis and trans nomenclature important in organic chemistry?
2 Answers
Jul 13, 2015
Because cis- and trans- compounds are really different.
Explanation:
Since a double bond can't rotate, the behaviour as to chemical reactions are quite different. An example (wikipedia):
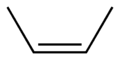
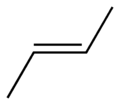
You can imagine that these two compounds would react quite differently to added functional groups, and that the end result of chemical reactions would be different.
Jul 24, 2015
Because they specify bond orientation, and double bonds are actually quite rigid. If you just say "2-butene", it doesn't say anything about the two geometric isomers of 2-butene, (E)-2-butene / cis-2-butene and (Z)-2-butene / trans-2-butene.


