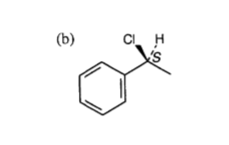
Why is configuration of this molecule S?
1 Answer
Jun 16, 2017
Because in terms of substituent priority,
Explanation:
Normally we would use a model to assign stereochemistry; however, here, the representation of the molecule aids our assignment, and we can make the assignment unambiguously.
The substituent of LEAST priority goes BACK in to the page, and this

