Why is electronegativity a factor that influences NMR spectra?
1 Answer
Electronegativity is an important factor in NMR spectroscopy because it affects the shielding of the nuclei.
Explanation:
When the electrons in a molecule are subjected to an external magnetic field
This induced field shields the nearby protons from the full force of
This effect is called diamagnetic shielding.
The nuclei experience only the effective field
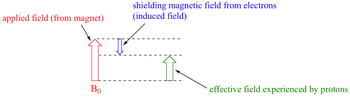
(from chemwiki.ucdavis.edu)
Their resonance frequency is slightly lower than what it would be if they did not have electrons shielding them.
An electronegative atom pulls valence electrons away from the atom and effectively decreases the electron density around the nuclei.
Thus, a lower value of
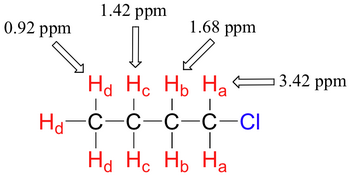
(from chemwiki.ucdavis.edu)
The
We see the same effect in
The carbon atom in ethane has a resonance frequency at δ 7 ppm.
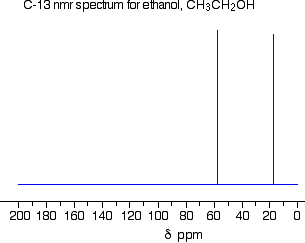
In ethanol, the electronegative

