Why is isotope notation important?
1 Answer
Isotopes are atoms of the same element that differ in the number of neutrons in their atomic nuclei. All atoms of the same element have the same number of protons, which is the atomic number of that element. However, because different isotopes have different numbers of neutrons, they can differ in mass number, which is the sum of the protons and neutrons in the nucleus.
Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words.
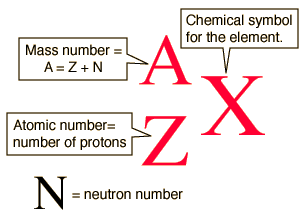
Additionally,
Example 1: What is the isotopic notation for the isotope carbon-14?
From the periodic table, we see that the atomic number (number of protons) for the element carbon is
We can determine the number of neutrons as
Example 2. Given the isotopic notation
a) Name of the isotope
b) Mass number
c) Atomic number
d) Number of protons
e) Number of neutrons.
Answers:
a) titanium-48
b)
c)
d)
e)

