Why is it that the more R groups there are around an empty orbital the more stable it is?
1 Answer
Alkyl groups stabilize an empty orbital by hyperconjugation.
Hyperconjugation is the delocalization of the electrons in a σ bond into an adjacent empty or partially filled orbital.
This forms an extended molecular orbital that increases the stability of the system.
Hyperconjugation explains why increasing the number of alkyl substituents on a carbocation makes it more stable.
Let's consider how a methyl group is involved in hyperconjugation with an empty p orbital

Note that the empty orbital is coplanar with one of the C-H sp³ σ-bonds.
This overlap interaction forms two new molecular orbitals.
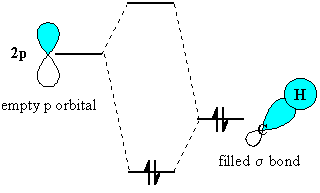
The two electrons go into the lower-energy molecular orbital, so the system is more stable.
The C-C σ-bond is free to rotate. As it does so, each of the C-H σ-bonds in turn undergoes the stabilizing interaction.
Thus, the ethyl cation has 3 C-H σ-bonds that can be involved in hyperconjugation.
The more hyperconjugation there is, the greater the stabilization of the system.
For example, the t-butyl cation has 9 C-H σ-bonds that can be involved in hyperconjugation. So (CH₃)₃C⁺ is more stable than CH₃CH₂⁺.


