Why is m-cresol soluble in Bicarbonate Solution?
1 Answer
Aug 12, 2018
Well, what is
Explanation:
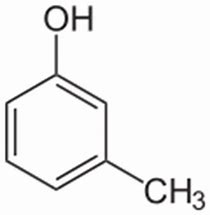
And thus m-cresol is a PHENOL derivative, that is much more acidic than an aliphatic alcohol. Its acidity,
And thus we expect the reaction with bicarbonate...

