Why is precipitation reaction consider as double displacement reaction explain?
1 Answer
Refer to the explanation.
Explanation:
A double displacement (double replacement or metathesis) reaction can have three possible products; a precipitate, an insoluble gas, or water (neutralization reaction). The solutions contain ionic compounds as solutes, and it is the ions that will react.
The general equation is:
where:
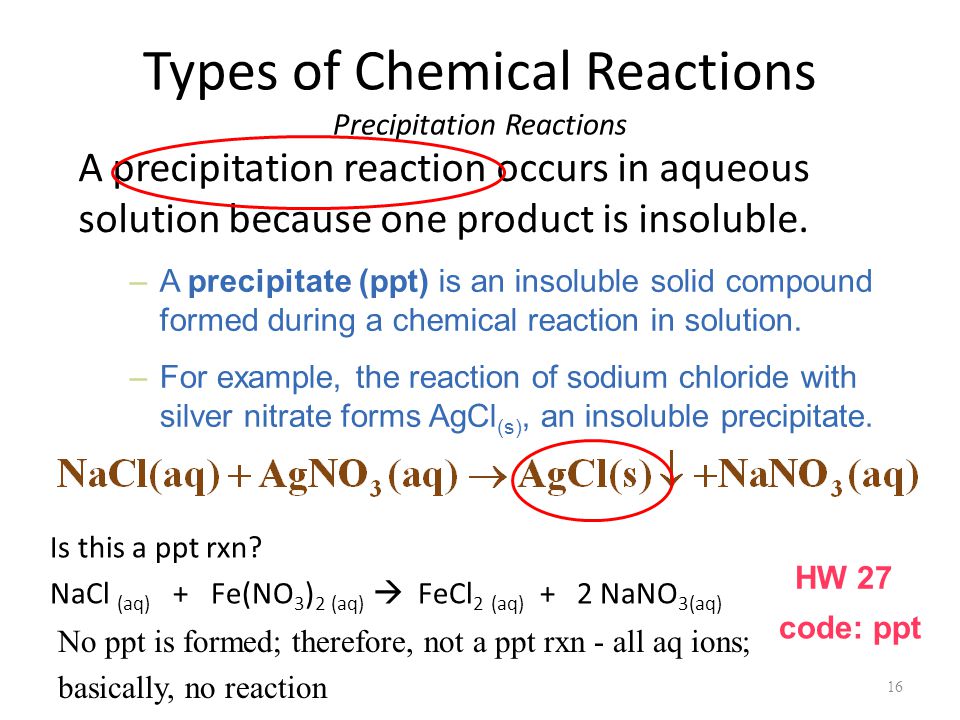
A precipitation reaction occurs when two solutions of ionic compounds are combined and they react to form an insoluble solid, which is the precipitate.