Why is respiration considered as an exothermic reaction?
1 Answer
Because the products are less energetic than the reactants.
Explanation:
In a combustion reaction, you typically tend to burn something in oxygen, e.g Butane:
A combustion reaction is undoubtedly exothermic as the products contain much less energy than the reactants.
Similarly in respiration we metabolize a carbohydrate (glucose in my example) together with oxygen to release energy:
Both of these reactions produce products which has a much lower chemical potential energy, so the rest of the energy is therefore released to the surroundings, making the reaction exothermic. They therefore have an enthalpy diagram that looks like this:
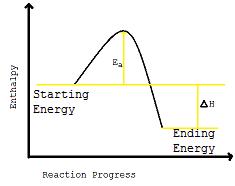
Which shows that some energy must be released from the reaction.

