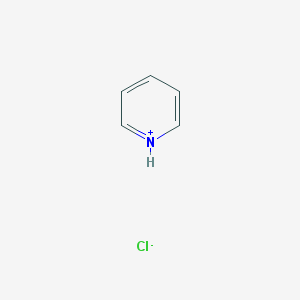
Why is the chloride ion not bonded to the pyridinium ion in pyridinium chloride?
1 Answer
Feb 23, 2018
Because pyridinium chloride is an acid base pair....
Explanation:
And we can see this from the synthesis reaction...
And so here chloride is the counterion of the pyridinium CATION...the interaction is ionic...and the nitrogen, as a cation, is quaternized....

