Why is the first ionization energy of aluminum lower than the first ionization of magnesium?
1 Answer
Jul 27, 2018
What are the electronic configurations of the aluminum and magnesium atoms respectively?
Explanation:
For
And for the reaction....
Now we assume that the valence electron is the electron that is ionized. A priori, would we not expect that a p-orbital electron would be more easily removed than a s-orbital electron, for the reason that the p orbital is FARTHER removed from the atomic nucleus, and has NO probability of occurring at the nuclear core.
But as chemists, as physical scientists, we should interrogate data...
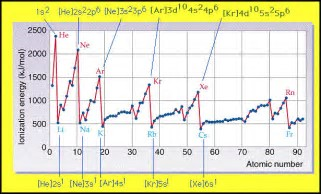
Does this table support our argument...?

