Why is the negative charge on the oxygen atom with the single bond?
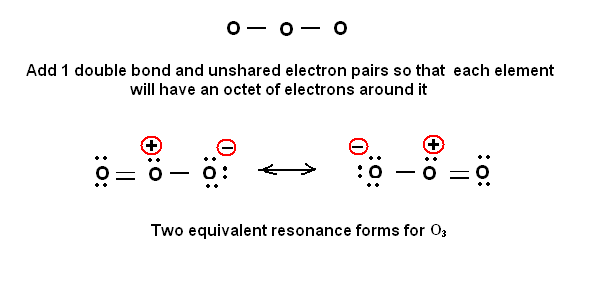
My question is that why is the negative charge on the oxygen atom with the single bond, why not on the oxygen atom with the double bond? I am confused.
My question is that why is the negative charge on the oxygen atom with the single bond, why not on the oxygen atom with the double bond? I am confused.
1 Answer
This is a good question, and illustrates the formalisms we use when we assign
Explanation:
Now ozone is a NEUTRAL molecule, and the Lewis structure indeed reflects this even tho there is charge separation. We follow the old convention that the TWO electrons of the covalent bond are SHARED EQUALLY between the atoms for the porpoises of charge assignment...
And so for
And now for
The electronic structure of ozone is thus based on a trigonal plane, but the molecular structure is BENT, with

