Why is the reaction of alkanes with iodine thermodynamically unfavorable?
1 Answer
Thermodynamically unfavorable means that
Recall the following equation:
#DeltaG = DeltaH - TDeltaS#
Now, recall the radical halogenation mechanism (for bromine, but it could be for any halogen because it's only theoretical):
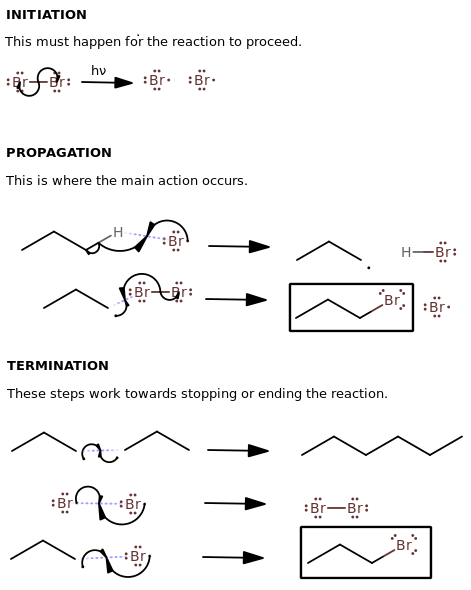
(Although the above mechanism uses bromine---I'm reusing one of my old images, you can replace it with iodine and it carries the same message.)
We should note that the reaction is thermodynamically unfavorable with iodine in the sense that the second step isn't able to go to completion.
THE PROPAGATION STAGE
Naturally, it has to do with how well the bond can form in the propagation stage.
First, note that the first bond broken is the
#DeltaH_("I"-"I", "break") ~~ "151 kJ/mol"#
#DeltaH_("C"-"H", "break", "CH"_4) ~~ "142 kJ/mol"#
#DeltaH_("H"-"I", "form") ~~ -"89 kJ/mol"#
As you can see, the total bond breaking/making enthalpy change is
However, entropy changes are actually very small for radical halogenation in general, meaning that
It could be that since radical reactions go until all reactants are used up, and are catalyzed by heat or light, it uses similar equivalents of reactants as it does products. Either way, the entropy change is close to
So, numerically, it turns out that
#DeltaG = ("+") - T("small") ~~ DeltaG#
What about pKa?
An additional way you could support this is that during the propagation stage, an
Why is the pKa so low? Probably because iodine is so large. The internuclear distance between hydrogen and iodide in hydroiodic acid is the largest of all the hydrohalogenic acids because iodide itself is pretty large (

