Why is water a good solvent?
1 Answer
Water is called the "universal" solvent because it can dissolve both ionic substances as well as polar covalent substances.
Explanation:
Because water
Water is a polar covalent molecule so it can dissolve other substances which are polar covalent such as sugars, alcohol, and most organic molecules.
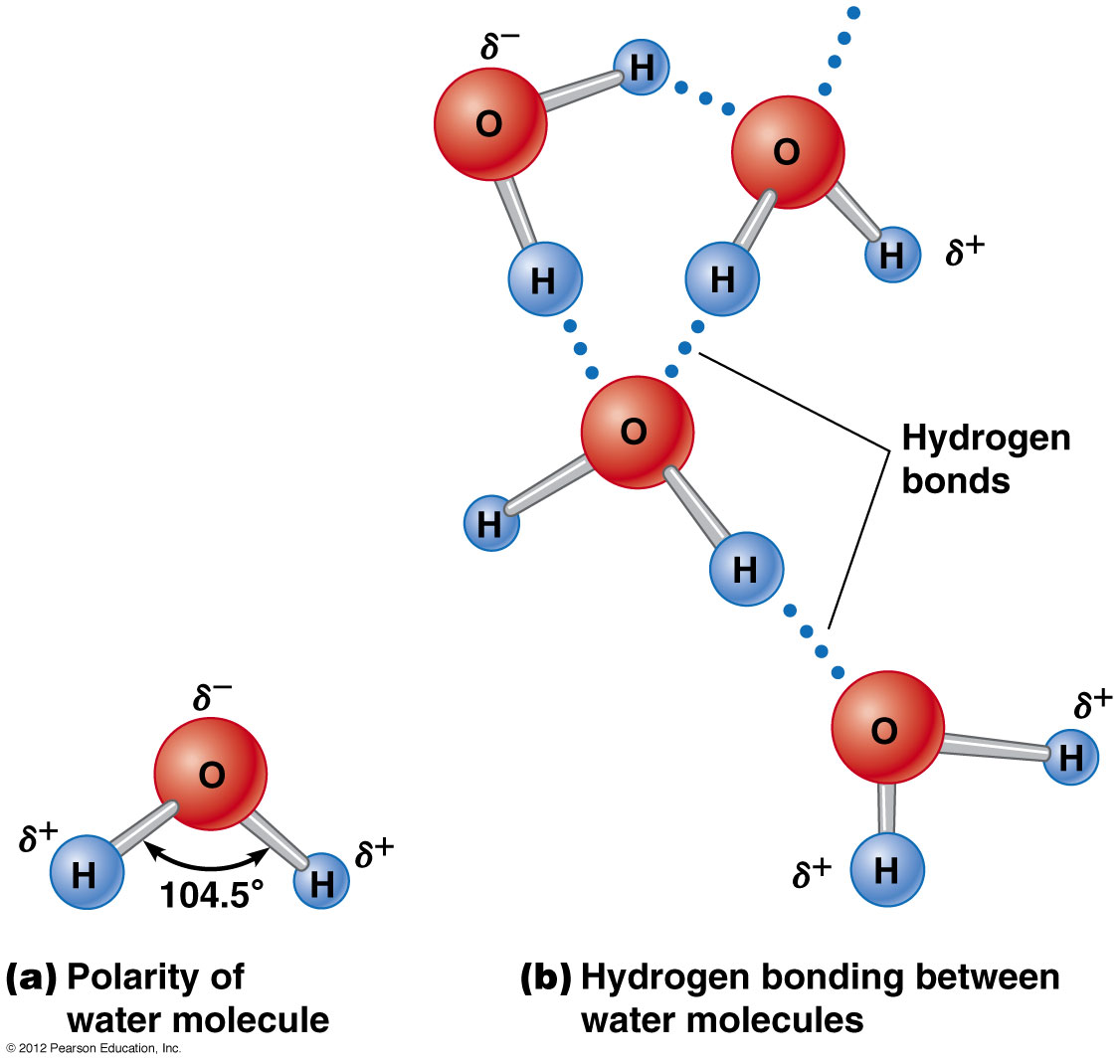
Water can not dissolve non polar molecules such as gasoline. However non polar substances are not common, meaning water can dissolve most substances.
Water also has a large liquid range of

