Why KMnO4 in alkaline solution accept 3e per Mn and act as mild oxidizing agent while in acidic solution can gain 5 electron per Mn act as strong oxidizing agen???
1 Answer
Consider the Pourbaix diagram of
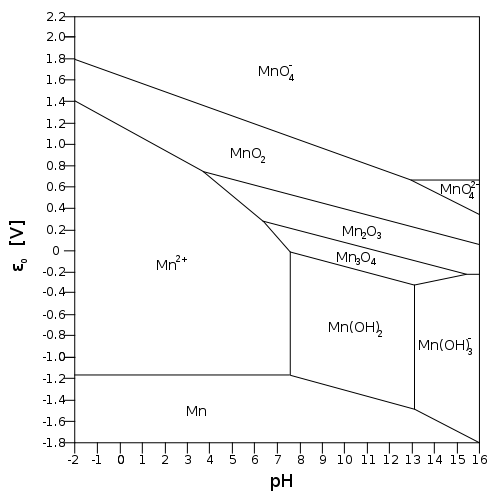
If it's easy to reduce
#stackrel(color(blue)(+7))"Mn""O"_4^(-)(aq) + 2"H"_2"O"(l) + 3e^(-) rightleftharpoons stackrel(color(blue)(+4))"Mn""O"_2(s) + 4"OH"^(-)(aq)#
#stackrel(color(blue)(+7))"Mn""O"_4^(-)(aq) + 8"H"^(+)(aq) + 5e^(-) rightleftharpoons stackrel(color(blue)(+2))("Mn"^(2+))(aq) + 4"H"_2"O"(l)#
In acidic solution, specifically below
The diagonal
#"MnO"_2(s) + 4"H"^(+)(aq) + 2e^(-) rightleftharpoons "Mn"^(2+)(aq) + 2"H"_2"O"(l)# The more acidic the
#pH# (the more#H^+# there is), the more the equilibrium shifts to#Mn^(2+)# by Le Chatelier's principle, favoring a#5e^(-)# reduction.
In basic solution (above
#"MnO"_2(s) + 2"H"_2"O"(l)(aq) + 2e^(-) rightleftharpoons "Mn"^(2+)(aq) + 4"OH"^(-)(aq)# And that shifts the equilibrium left, towards
#stackrel(color(blue)(+4))(Mn)O_2(s)# , favoring a#3e^(-)# reduction.
And so, in basic solution, we only have a change in oxidation state of

