Why magnesium alkoxide is called complex as it don't consist any transition element?
1 Answer
Magnesium alkoxides are called complexes because they often exist as complex "clusters" of the alkoxide.
Explanation:
Alkoxide ions are good bridging ligands, that is, they can form bonds to two or more metal ions.
You see this, for example, in the dimer of
where
Thus, four magnesium methoxide units combine in solution to form cubic structures of
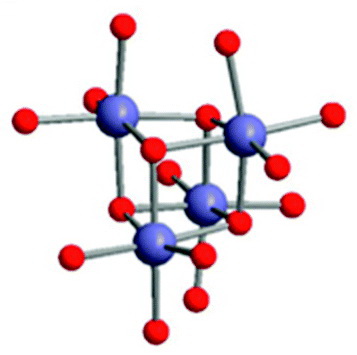
(Adapted from [ RSC ] Publishing - Royal Society of Chemistry)
The diagram shows only the
One of the
Two methanol molecules are also coordinated to each

