Why might a bright yellow solid form when two clear, colorless liquids are mixed?
1 Answer
A precipitate is formed as a result of a double displacement reaction.
Explanation:
When two substances (can be aqueous or liquids) in solution react, they yield a solid and an aqueous compound.
This is a double displacement reaction in which the solid is known as a precipitate.
You can determine which compound is the precipitate by using a solubility chart.
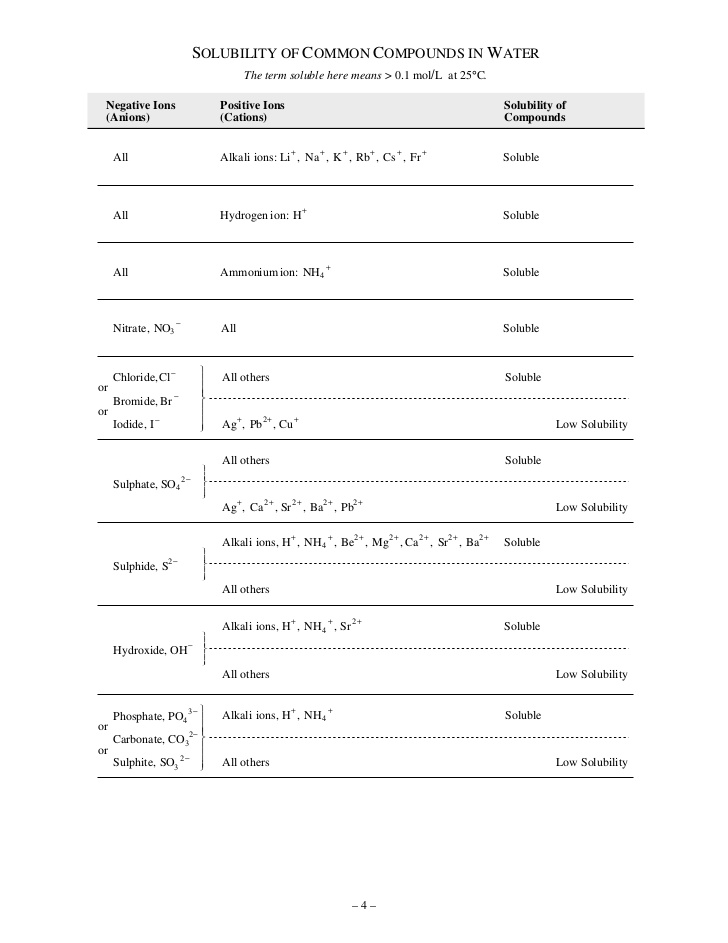
Soluble =>
Low solubility => precipitate will form
An example of a yellow precipitate forming is through the reaction of potassium iodide,
The products are lead (II) iodide,

Notice how both solutions (before reaction) are colourless. The solution in the person's hand is colourless and the solution in the other beaker is colourless (before reaction).
We can use the solubility chart to determine the precipitate.
We see that for the first product
Hope this helps :)


