Why noble gases are sparingly soluble in water ?
1 Answer
They are non-polar.
Explanation:
Noble gases are non-polar substances, so the only form of intermolecular forces that they have are London dispersion forces.
When a polar molecule (with dipole-dipole forces) dissolves in water, the slightly positive end of water is attracted to the slightly negative end of the other molecule.
So, the molecules will be separated from each other because they have a greater attraction with water.
However, when noble gases are dissolved in water, they only dissolve very slightly.
Noble gases only have London dispersion forces, so there's very little attraction between water and these noble gases.
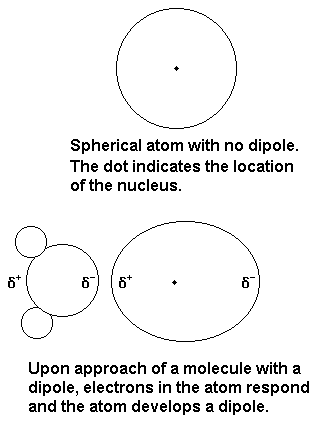
They still can dissolve, but only very little, because some water molecules will be able to form dipole-induced dipole attractions with the noble gas atom.
This is when the positive side of a water molecule induces a negative charge on the noble gas atom (or vice versa), creating a slight attraction.