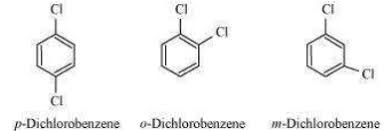
Why p-dichlorobenzene has higher melting point and lower solubility 5han those of ortho and meta of p-dichlorobenzene ??
1 Answer
Jun 18, 2018
p-dichlorobenzene is the most symmetrical structures of it's isomers.( melting point increases with increase in symmetry.)
it fits most closely in the crystal lattice and hence has strong intermolecular bonds.
so it needs a lot of energy to break this bond
hence it has lower solubility.