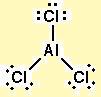
Why is there an empty #p# orbital in #"Al"# of #"AlCl"_3?#
1 Answer
Nov 26, 2017
Per the literature,
Explanation:
The extra p-orbital is simply not included in the hybridization needed to attach 3 substrates to the central element, Al. Only an s and 2 p-orbitals are needed.