Why transition elements show tendency to form large number of complexes??
2 Answers
It is because the transition metals have variable oxidation states.
Explanation:
The transition elements span from group 3 to 11.They show variable oxidation states according to the catalyst, reacting element or compound, and the conditions of the reaction they are participating in.Thus, they can form a large number of complex compounds
They also form coordination compounds which have
It's because they have
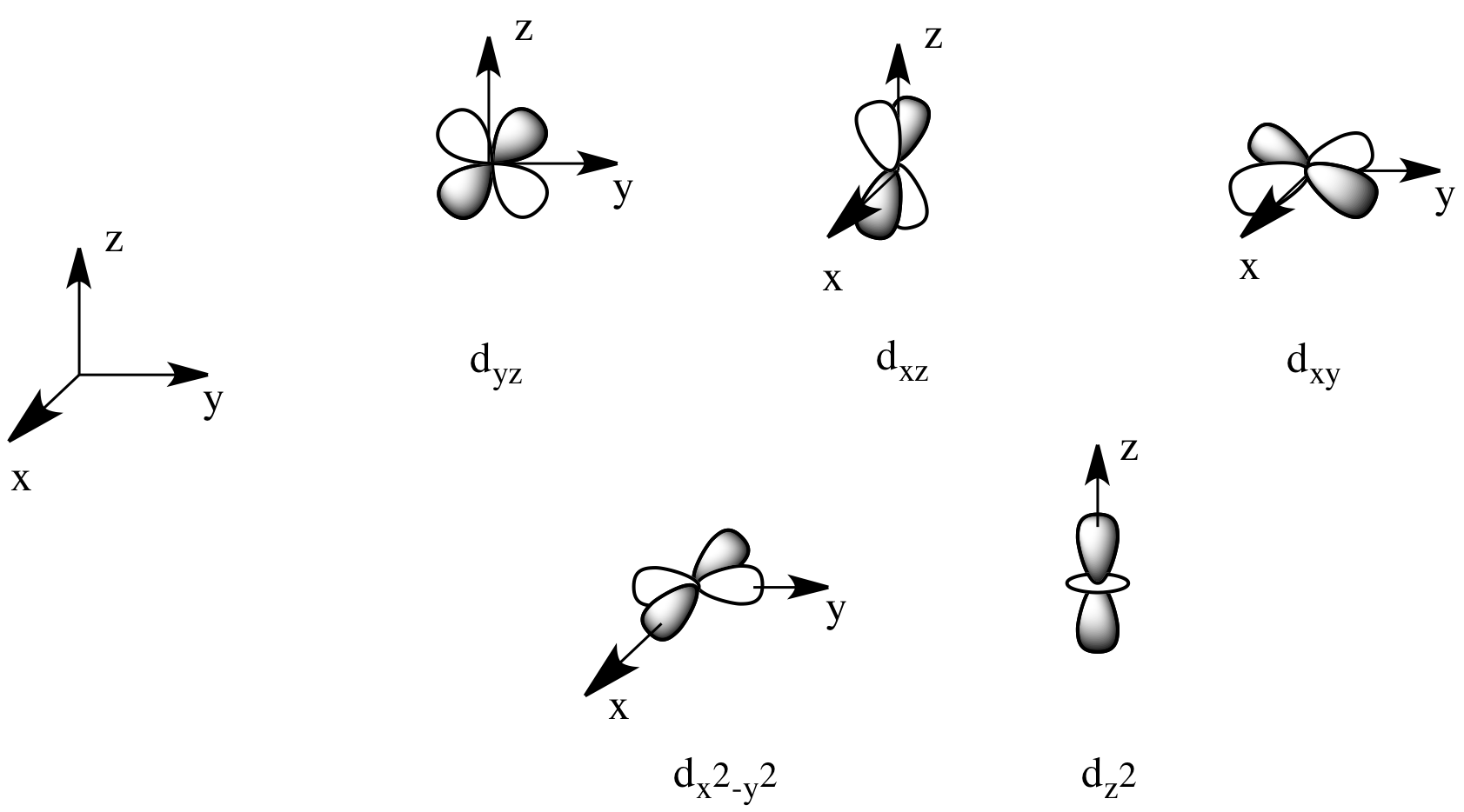
Some examples...
- The
#d_(z^2)# and#d_(x^2 - y^2)# are useful for#sigma# bonding along the coordinate axes.
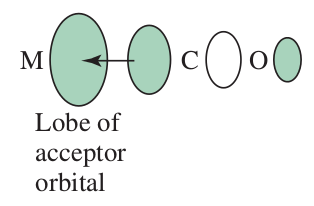
- The
#d_(xy)# ,#d_(xz)# and#d_(yz)# can be used for#pi# bonding on octahedral complexes.
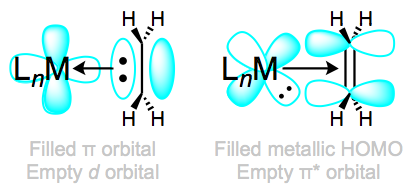
The right-hand side of the diagram illustrates
#pi# bonding with ethene using a#d_(xy)# orbital, and the left-hand side is a#sigma# bond with ethene using a#d_(x^2-y^2)# orbital.This kind of bond is known to rotate at low temperatures, and thus, variable-temperature NMR is useful in identifying such complexes.


