Why when i add #NaOH# to 1 it give 2 and not 3 ?
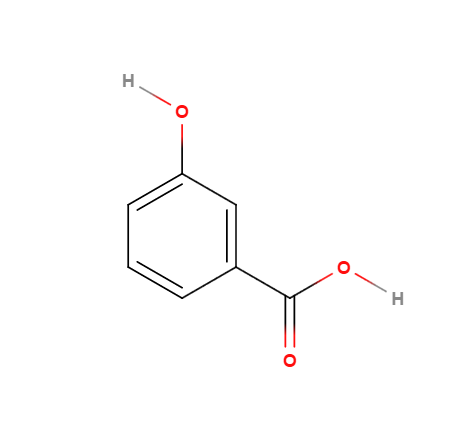
(1)
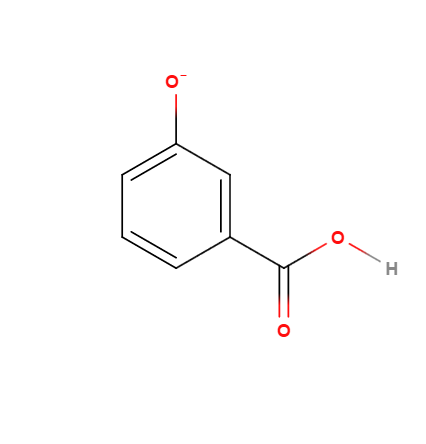
(2)
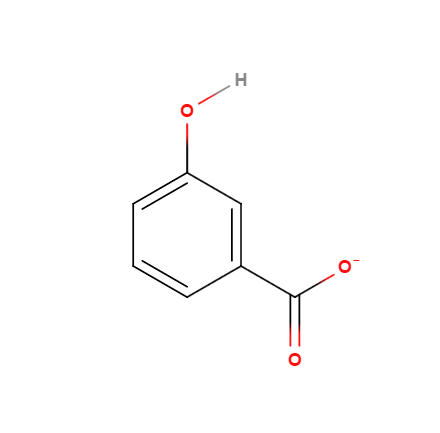
(3)
Carboxylic acid a more acid than alcool so why i have this result ?
(1)
(2)
(3)
Carboxylic acid a more acid than alcool so why i have this result ?
1 Answer
It could be explained by steric hindrance.
Explanation:
In this case I would question the original question premise. A carboxylate ion is much more stable than the corresponding alkoxide ion because of the existence of resonance structures for the carboxylate ion which disperse its negative charge. https://www.britannica.com/science/carboxylic-acid
Thus, #3 would be the expected result.
To argue for steric hindrance, the ability of the molecule to form an ion that will essentially be "balanced" by a free
Thus, the alcohol group would more susceptible to reaction in this molecule. However, as stated initially – the stability of the carboxylate ion compared to the alkoxide ion would most likely result in the last structure, not the second one.

