Why xenon being a noble gas forms compounds with other elements?
1 Answer
Xenon forms compounds because its inner electrons screen the outer electrons from the nucleus.
Explanation:
Its outer electrons then become a target for other highly electronegative atoms.
Sir William Ramsay received the 1904 Nobel Prize for Chemistry for discovering the "noble gases".
Chemists originally believed that the noble gases could not form compounds , because their full valence shell of electrons made them chemically stable and unreactive.
But the heavier noble gases have more electron shells than the lighter ones.
The inner electrons shield the outermost electrons, so they are less strongly attracted to the nucleus.
A highly electronegative element such as fluorine can, at least, share these electrons.
In 1962, Neil Bartlett prepared the first noble gas compound. That same year, other chemists prepared
Since then, many other noble gas compounds have been synthesized.
Here are just some of the xenon compounds:
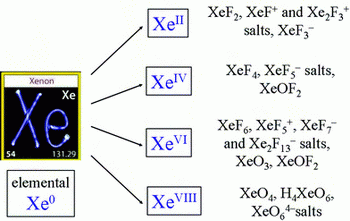
(from chemistry.stackexchange.com)

