Is this molecule acidic or basic?
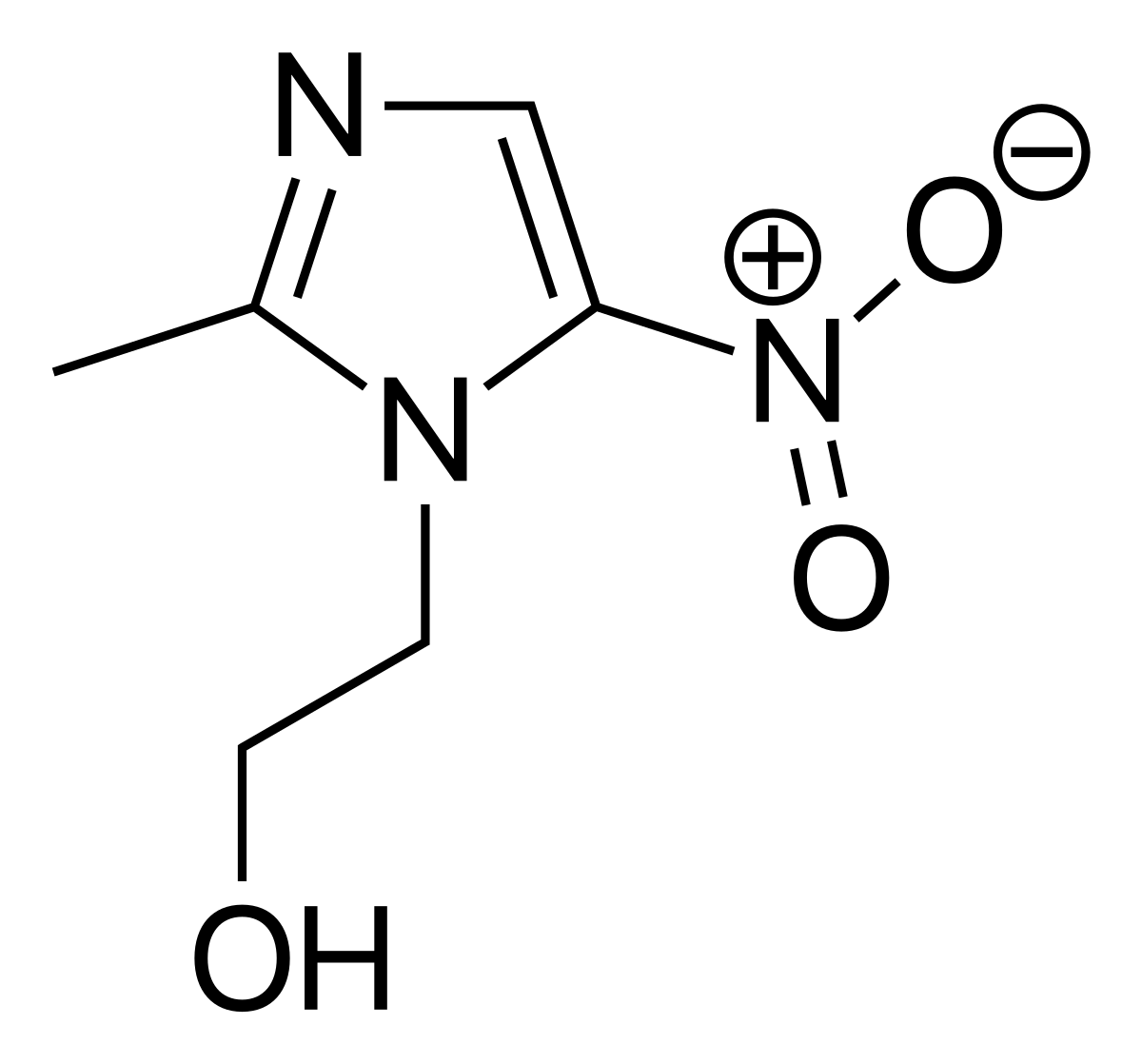
A)Metronidazole
B) Which group will more readily undergo hydrolysis-ester or carbonate if they are in the same molecule?

A)Metronidazole
B) Which group will more readily undergo hydrolysis-ester or carbonate if they are in the same molecule?
1 Answer
Oct 21, 2017
Basic.
Explanation:
This is where the Lewis Acid-Base definition becomes useful. A Lewis acid is a proton donor, and a base is a proton acceptor. The given molecule is most likely to accept a proton, and is therefore a Lewis Base.

