Write a stepwise mechanism that shows how a small amount of CH3CH3 could form during the bromination of CH4?
1 Answer
Here is the mechanism drawn out. I rectangled where the ethane gets produced.
Something to keep in mind here is to not get so caught up about the actual equiv. of molecules depicted here. These steps in the mechanism are not about keeping track of how many molecules are unreacted or reacted, but about tracking what kinds of key steps occur that describe the key points of the reaction.
INITIATION STEP
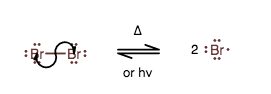
Tracing this mechanism, the initiation step homolytically cleaves the
PROPAGATION STEPS
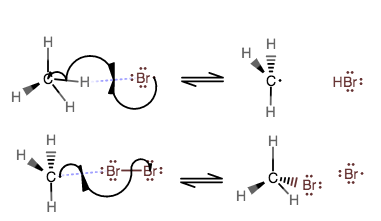
Then, one of them reacts with methane to heterolytically cleave it and form one equiv. of a methyl radical and
Another equiv. of
Now, since at some point in the propagation steps, a methyl radical gets formed, if you have enough equiv. of each reactant, there is a chance that two methyl radicals will react in the termination steps, and at that point, you will form ethane.
This amount is small simply because in the actual propagation steps, it is depicted that the methyl radical that forms is supposed to react again with
TERMINATION STEPS
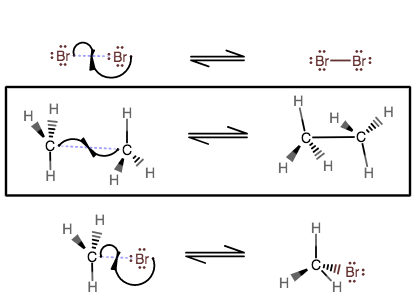
The two equiv. of bromine radicals react to reform

