Write complete mechanism of dehydration of 2-methyl-1-pentanol?
1 Answer
See below.
Explanation:
You can draw this out in MarvinSketch, for example, as:
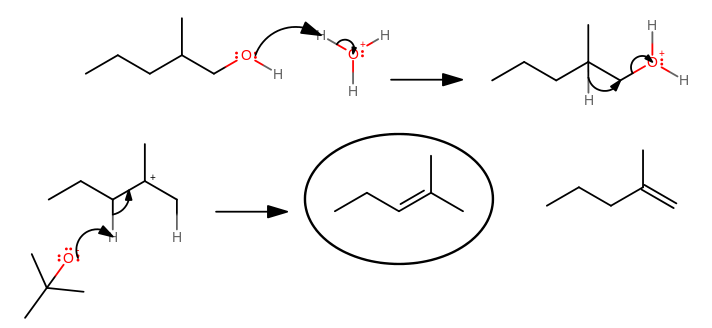
- The hydroxyl group by itself is not a good leaving group, but protonating it makes it a great leaving group.
- The 1,2-hydride shift occurs to achieve a more stable carbocation intermediate.
For primary carbocations that would have been formed, the hydride shift occurs with the departure of the leaving group in one step to avoid forming such an unstable cation; for higher-substituted carbocations, this occurs more often in two steps.
- The
#beta# -elimination occurs between the base and either of the protons antiperiplanar to the cationic carbon center (left proton gives major product, right proton gives minor product)
The circled product is the major product according to Zaitsev's Rule of achieving a more substituted alkene. An


