Write the formula of the simplest chloride you would expect the element of atomic number 33 to form? Draw a diagram showing the bonding present in this chloride.State the sape of this chloride molecule?
2 Answers
As number 33 would form a compound
Explanation:
As is in the same family as N which is able to form three bonds.
The outer valance shell of As is
The shape of the molecule will be triangular pyramidal.
The four orbitals of As will form a tetrehedral shape. The unpaired electrons will occupy three of the four points creating a triangle.
The one set of paired electrons will occupy the fourth point. This will create the shape of a pyramid with a triangular base.
Here's what I get.
Explanation:
Element 33 is arsenic. It is in Group 15, so it needs three electrons to complete its octet.

The
We can represent this structure more compactly as a Lewis structure:
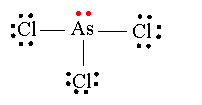
The lines represent the shared pairs of electrons.
According to VSEPR theory, the four electron domains around the
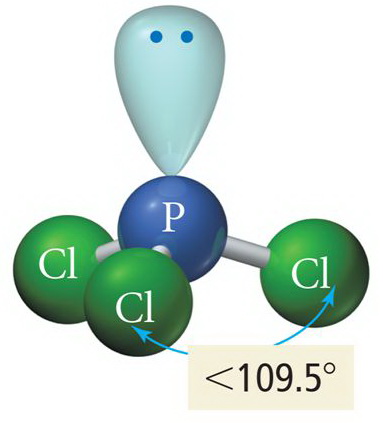
(Adapted from SlidePlayer)
However, we can't"see" the lone pair, so we say that the molecular shape of


