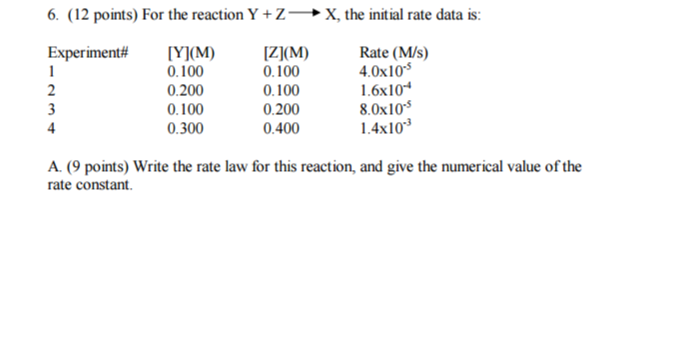
Write the rate law for this reaction, and give the numerical value of the rate constant?
What will the observed initial rate be if the initial concentration
[Y]=0.400 and [Z]=0.300?

What will the observed initial rate be if the initial concentration
[Y]=0.400 and [Z]=0.300?
1 Answer
The rate law in general just relates the rate
#r(t) = k[Y]^m[Z]^n# where
#m# is the order of reactant#Y# and#n# is the order of reactant#Z# . We do not know#m# or#n# yet, so we must find those to finish writing the rate law.
RATE LAW ORDERS, AND RATE LAW
To make our lives easier, let us set
#r_(i,1)(t) = k[Y]_(i,1)^m[Z]_(i,1)^n#
#r_(i,2)(t) = k[Y]_(i,2)^m[Z]_(i,2)^n#
But since
#(r_(i,2)(t))/(r_(i,1)(t)) = (cancel(k)[Y]_(i,2)^mcancel([Z]_(i,2)^n))/(cancel(k)[Y]_(i,1)^mcancel([Z]_(i,1)^n))#
#(r_(i,2)(t))/(r_(i,1)(t)) = ([Y]_(i,2)^m)/[Y]_(i,1)^m#
#(1.6xx10^(-4))/(4.0xx10^(-5)) = ("0.200 M"/"0.100 M")^m#
If you do the math, you'd get a comparison:
#4 = 2^m#
Thus,
For
#(r_(i,3)(t))/(r_(i,1)(t)) = ([Z]_(i,3)^n)/[Z]_(i,1)^n#
#(8.0xx10^(-5))/(4.0xx10^(-5)) = ("0.200 M"/"0.100 M")^m#
If you do the math, you'd get a comparison:
#2 = 2^m#
Thus,
Therefore, the overall rate law is:
#color(blue)(r(t) = k[Y]^2[Z])#
RATE CONSTANT
The rate constant is specific to the reaction, not to the experiment. That means we can pick any rate from any trial, and find the rate constant for the entire reaction.
#r_(i,1)(t) = k_1[Y]_(i,1)^2[Z]_(i,1)#
#4.0xx10^(-5) = k_1("0.100 M")^2("0.100 M")#
#=> color(blue)(k_1) = (4.0xx10^(-5) "M/s")/(("0.100 M")^2("0.100 M"))#
#= color(blue)("0.04 M"^(-2)cdot"s"^(-1))#
To prove that
#r_(i,2)(t) = k_2[Y]_(i,2)^2[Z]_(i,2)#
#1.6xx10^(-4) = k_2("0.200 M")^2("0.100 M")#
#=> color(blue)(k_2) = (1.6xx10^(-4) "M/s")/(("0.200 M")^2("0.100 M"))#
#= color(blue)("0.04 M"^(-2)cdot"s"^(-1))#
Thus,
At this point you should be able to finish the problem yourself. Once you have the rate constant, you can use your given concentrations in the last part of the problem to find the initial rate of the reaction that corresponds to those new concentrations.

