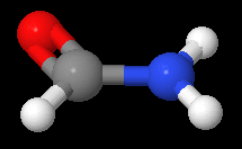
Write the state of hybridization of carbon in following compounds and shapes of each of the molecules (a) CH3Cl (b) HCONH2 ?
1 Answer
(a)
Explanation:
You must draw the Lewis structures, use VSEPR theory to determine the electron geometry and shapes of each molecule, and then assign the hybridization that corresponds to the electron geometry.
(a)
The structural formula is
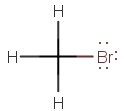
The central atom (
(
Per VSEPR theory, the electron geometry and the molecular geometry are each tetrahedral.
A tetrahedral electron geometry corresponds to
(b)
The Lewis structure for formamide is.
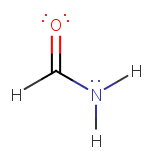
The
Thus, the