Here is a short pK_"a" table.
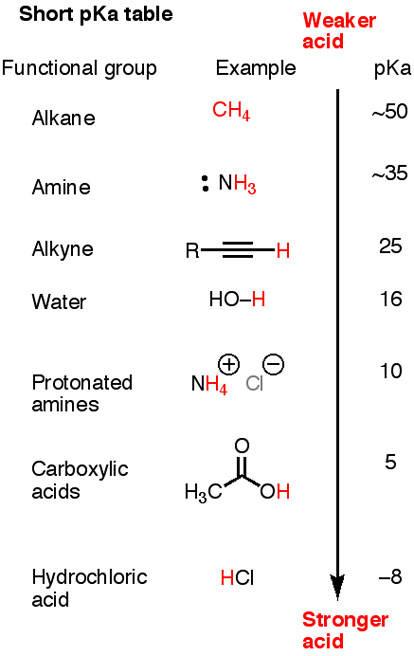
Note that "HC≡C-H", "H"_2"N-H", and "H"_3"C-H" are all weaker acids than water.
The corresponding conjugate bases, "H-C≡C:"^"-", "H"_2"N:"^"-", and "H"_3"C:"^"-", are strong. They will all deprotonate water.
The conjugate base of methane "H"_3"C:"^"-" is strong enough to deprotonate anything below it in the table.
Methyllithium, "CH"_3"Li", is one of the strongest bases around.
Acetylide ion ("H-C≡C:"^"-") is strong enough to deprotonate only an acid with a "pK"_"a" < 25.
Acetate ion ("CH"_3"COO"^"-") is weaker still, able to deprotonate only an acid with a pK_a < 5.
A more complete table of pK_"a" values will give you many more conjugate bases that will deprotonate water.


