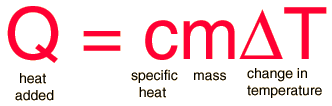Question #362ab
1 Answer
Apr 16, 2014
Specific heat capacity, ice: 2.108 kJ/kgK
Specific heat capacity, water: 4.187 kJ/kgK
Specific heat capacity, water vapor: 1.996 kJ/kgK
We are talking here about different
The specific heat is the amount of heat per unit mass required to raise the temperature by one degree Celsius. The relationship between heat and temperature change is usually expressed in the form shown below where c is the specific heat. The relationship does not apply if a phase change is encountered, because the heat added or removed during a phase change does not change the temperature.