Is the molecule #SF_4# polar or non polar?
1 Answer
The easiest way to determine if a molecule is polar or nonpolar is to draw its Lewis Structure and, if necessary, check its molecular geometry.
Explanation:
If there is an odd number of lone pairs of electrons around the central atom, the molecule is polar.
If there is an even number of lone pairs, you must check the VSEPR structure to decide.
In your example of

You can see that there is a lone electron pair around the sulfur atom and thus, the molecule is polar! :)
Here's why the "odd number of lone pairs" method works.
In VSEPR theory, the lone pair forces the molecular geometry of
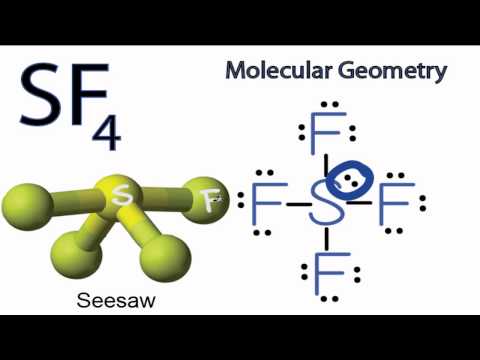
Two of the S-F bonds are pointing away from each other, and their bond dipoles cancel.
But the other two
Their bond dipoles do not cancel, so the molecule is polar.
On the other hand,
This is an even number, so you have to check the shape of the molecule.

Here, the
Hope this helps!


